Conductivity of Semiconductors
Now let’s consider the conductivity of semiconductors.
Consider silicon which, like carbon, has the diamond cubic cyrstal structure. The valance electrons are all covalently bonded in sp3
orbitals. These orbitals are completely filled. However, in this case,
the next available energy level (in the Conduction Band) is 1.1 eV
above the highest occupied level.
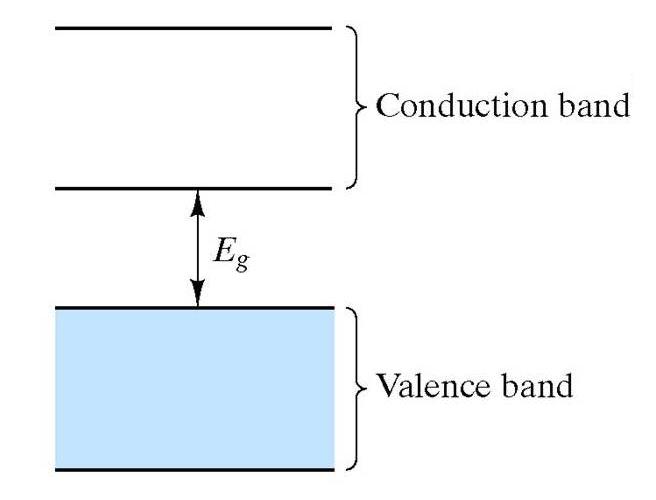
So
the picture is exactly the same as that for the insulating materials,
except that the the size of the energy gap is smaller. Hence
for Si, with an energy
gap of 1.1 eV, at room temperature some electrons will be promoted into the conduction band
which accounts for its intermediate value of conductivity. Furthermore,
for every electron promoted into the conduction band, a "hole" is
left in the valance band!
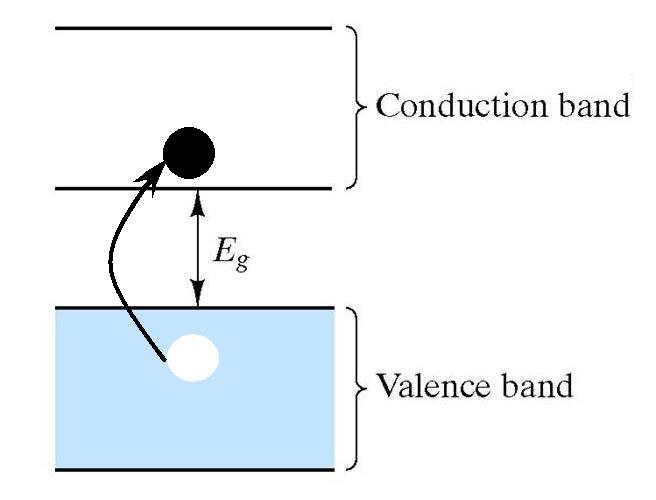
These
holes are also considered to be charge carriers. So there are two types
of carriers for electrical conduction: electrons and holes. Electrons
are called n-type carriers (for negative) and holes are called p-type carriers (for positive).
For intrinsic semiconductors (no impurities), the number of electrons will be equal to the number of holes.
ne = nh
Thus, the conductivity for an intrinsic semiconductor can be calculated:
sigma = ne q me + nh q mh
And since ne = nh the conductivity for an intrisic semiconductor is given by this equation:
sigma = ni q (me + mh)
where i stands for intrinsic carrier concentration.
Overall Conclusion on the Conductivity of Semiconductors:
Semiconductors
are semi-good electrical conductors because although their valence band
is completely filled, the energy gap between the valance band and the
conduction band is not too large. Hence some electrons can bridge it to become charge
carriers. The difference between a semiconductors and an insulator is the magnitude of the energy gap. For semiconductors Eg < 2eV and for Insulators Eg > 2eV.
